|
|
|
It's The Waterand a lot more
Easily overlooked and often misunderstood, the chemistry in the water we use in a cooling system is becoming more important all the time. As engine designs become more sophisticated, so does the coolant mixture requirements that are used. Since a 50/50 mixture of coolant is half water, dissolved minerals and chemicals in the water we use have the potential to interfere with the intentions of the inhibitors in the coolant. It should be no surprise to anyone that tap water varies from place to place. What you may not know is that depending on which part of the world you are from, the tap water varies even more significantly.
Commonly held thoughts regarding specifications for tap water when used in a coolant mixture for automotive uses:
It has been widely suggested that if your tap water does not meet the above minimum standards, then distilled water should be used. Conventional wisdom also suggests that 'Spring Water" or 'Drinking Water' should be avoided, and 'Well Water' should never be used under any circumstance.
With the purity of water such a wildcard, it is not surprising that the trend of packaging automotive coolant is in the "Ready to Use" or pre-diluted form. Currently both Toyota and Honda offer their own brand of premixed coolant. European and American OE manufactures' have so far resisted the pre-diluted trend, and still offer concentrated coolant.
Independent coolant packagers (Prestone, etc.) are on both sides of the fence, offering both concentrated and pre-diluted coolants. However, the trend is changing, depending on the particular store, it is now easier to purchase pre-mixed coolant (50% water) than the traditional concentrated coolant (0% water).
Water, in its chemically pure state is nearly a perfect electrical insulator. It is however, highly polarized which can make very strong electrolytic solutions conductive enough to carry significant electrical currents. Water has long been called the universal solvent which means it will easily dissolve other substances and is often a benchmark characteristic.
Distilled Water is produced by first boiling or evaporating water, then concentrating and condensing the water vapor back to a liquid state. This form of water purification is excellent for removing minerals, chemical contaminates, and viruses. The distillation process takes a significant amount of energy since the water must change states twice. Bio-chem Laboratories might actually double distill water when making ultra pure processes. Distilled water is generally held to be ideal for use in an automotive cooling system as it is very pure and not capable of conducting electricity. However, this extra purity may in fact point us in the wrong direction when dealing with the challenges of automotive electrolysis. Pure water will adapt to the environmental conditions around it, and may act to enhance the galvanic process (electrolysis). Since water is rather good at dissolving things ( There is a reason it's called The Universal Solvent ) pure water will reach back into the water jackets of the engine block and absorb sediments that were once dormant. With no other dissolved minerals to fight it, this pure water has unwittingly now become part of the problem. That is why I characterize distilled water as a double agent: It is great in a new clean maintained cooling system. However distilled water is not the right choice when our cooling system presents symptoms of chronic galvanic cell corrosion (electrolysis).
Deionized Water is purified water that has passed through a ion exchanger resin which exchanges a hydrogen ion and hydroxide ion for dissolved minerals. Deionized water is not as pure as distilled water, but is easier, faster and cheaper to produce. Di water is pure enough for a wide variety of uses including cooking and drinking, however bacteria and virus's can still be present in deionized water. For automotive uses deionized water is ideal for topping lead acid batteries, and mineral free exterior washing. Deionized water is sometimes also named and marketed as de-mineralized water. Deionized water almost has no place inside the automotive cooling system, as the mineral free properties may work against us just as in the distilled water. Using Di water for general maintenance when there are no symptoms of galvanic corrosion might be ok, but using DI water when a cooling system is infected
Reverse Osmosis Water systems purify water by using a number of steps. First the water is filtered down to remove rust and other sediments, then a smaller filter to remove even more contaminates. Usually at this point an activated charcoal filter is introduced to remove chlorine and other organics that will interfere with the RO process. Next the pressurized water is in contact with a thin film membrane that further purifies the water by allowing osmosis. Once the water has migrated beyond the membrane, it again has contact with an activated charcoal filter that removes any particles left over from the previous steps. UV light is sometimes exposed to the water in some cases if the need dictates. Smaller RO water process use no electricity and the water does not change states. No pumping is needed when the beginning water pressure is sufficient. Typical smaller RO water units produce 2-5 gallons of water a day, making them ideal for home or office water needs. However, smaller household RO units are not very water efficient and can use (reject) 15 gallons of water for every gallon they make. RO units are often coupled with soft water systems to remove the sodium chloride ions left behind by the soft water process.
Chlorinated Water is a problem for our automotive cooling systems. Chlorine and Chloramines are added to our public drinking water supply to disinfect the water from pathogenic disease. While disease free drinking water is the desired result, the chlorine charge creates problems for our metals. The Chlorine is a nonmetallic Anon, it reacts with the Aluminum in the cooling system which is a Cation. Since the electrostatic attraction between the positives and the negatives bring the particles together, we now have an ionic compound.
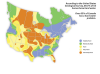
Hard Water is water that has higher than normal concentrations of dissolved minerals, typically calcium and magnesium. While these are not considered harmful to human health, these minerals can play havoc on modern household plumbing as the minerals tend to fall out of suspension and adhere to the insides of certain types of pipe. Soft water systems have a resin that exchanges sodium ions for calcium and magnesium ions, allowing the water to be "soft". Softened water is not good in an automotive cooling system, and a known good source of water is advisable over soft water. Well water and/or ground water is generally known for having a higher than normal "hardness" factor as well as iron and sulfate content and should be avoided in the automotive cooling system.
Tap Water can also sometimes be harmful to an automotive cooling system if the levels of dissolved solids and chlorine are high enough. The Chlorine ions can be very reactive and corrosive to aluminum and other metals including stainless steel. When Chlorine is added to water, it reacts to form a pH dependant equilibrium mixture of Chlorine, Hypochlorous Acid, and Hydrochloric Acid (CL2 + H2O -> HOCL + HCi). Additionally, naturally occurring Sulfates (SO4) found in tap water are generally not removed by public water systems and can also contribute to rapid metal failure. Reverse Osmosis and Distillation water treatments containing an activated charcoal stage are very effective at removing Chlorine, Chloramines, and Sulfates and is a great final stage treatment for any city water used for drinking, cooking, or any automotive use.
Properties of Water
|
|
|
It's the water
And a lot more...
|
|
|||||
 |
|
 |
|||
|
|